Exciting developments are unfolding in the world of neural technology! The potential to directly interface with the brain holds immense promise, and recent news highlights the progress being made in this groundbreaking field. It’s a thrilling time to witness these advancements, as they could revolutionize how we treat neurological conditions and enhance human capabilities.
Neuralink Chip Recipient Experiences Positive Changes
Reports are surfacing that an individual implanted with a Neuralink brain chip is already experiencing positive changes. While specific details are still emerging, the implications are significant. Imagine the possibilities: individuals regaining lost motor skills, improved communication for those with speech impairments, and even a deeper understanding of the brain itself. The journey is just beginning, but these initial findings offer a glimpse into a future where neurological challenges can be directly addressed through innovative technology. It sparks a hope for a better tomorrow, paving the way to a future where the impact of neurological conditions might be substantially reduced. I look forward to seeing the continued refinement and evolution of Neuralink’s technology, and the impact it has on those with disabilities.
Neuralink Receives FDA Approval for Second Patient Implant

The US Food and Drug Administration (FDA) has granted Neuralink clearance to implant their brain chip in a second patient, as reported by the Wall Street Journal. This is a major milestone for the company and the field of neural technology as a whole. Securing FDA approval is a rigorous process, requiring extensive data and demonstrating safety and potential efficacy. This second approval suggests that Neuralink is making significant strides in meeting regulatory requirements and advancing their technology responsibly. This news further fuels the excitement and anticipation surrounding Neuralink’s work. Having a second implanted patient will allow for a broader base of research, and will allow the company to adjust their approach based on results. It’s a chance to enhance the technology, improve user experience, and build confidence in the safety and utility of the implant. Every successful step is a step closer to realizing the transformative potential of this technology.
If you are searching about Neuralink gets approval to put brain chip in humans | Information Age | ACS you’ve visit to the right page. We have 10 Images about Neuralink gets approval to put brain chip in humans | Information Age | ACS like Human with Neuralink brain chip sees improvement after initial, Neuralink's brain chip is ready for human trials | GINX Esports TV and also Neuralink gets approval to put brain chip in humans | Information Age | ACS. Here you go:
Neuralink Gets Approval To Put Brain Chip In Humans | Information Age | ACS
ia.acs.org.au
Elon Musk's Neuralink Shows First Brain-chip Patient Playing Chess

news.sky.com
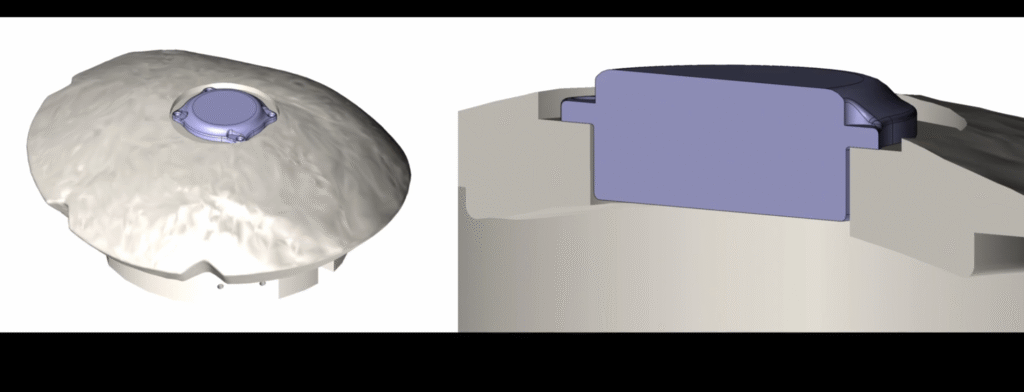
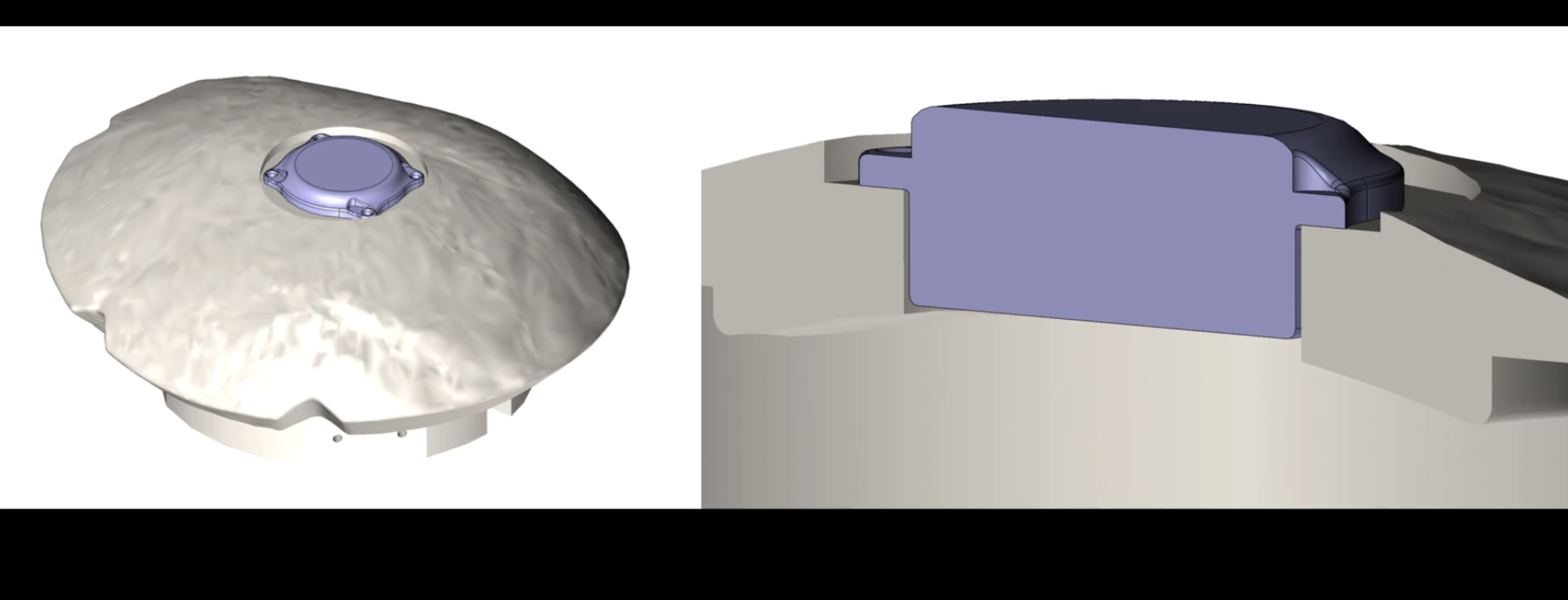
Here's How Neuralink Is Modifying Its Brain Chip For Second Human Patient

au.pcmag.com
Human With Neuralink Brain Chip Sees Improvement After Initial
ca.news.yahoo.com
Here's How Neuralink Is Modifying Its Brain Chip For Second Human Patient
au.pcmag.com
Neuralink Chip In Brain Microchip Head Royalty Free Vector
www.vectorstock.com
Neuralink Brain Chip: What Is It And How It Works | Technology

www.timesnownews.com
US FDA Clears Neuralink's Brain Chip Implant In Second Patient, WSJ

www.reuters.com
Neuralink Has Put Its First Chip In A Human Brain. What Could Go Wrong

lens.monash.edu
Neuralink's Brain Chip Is Ready For Human Trials | GINX Esports TV
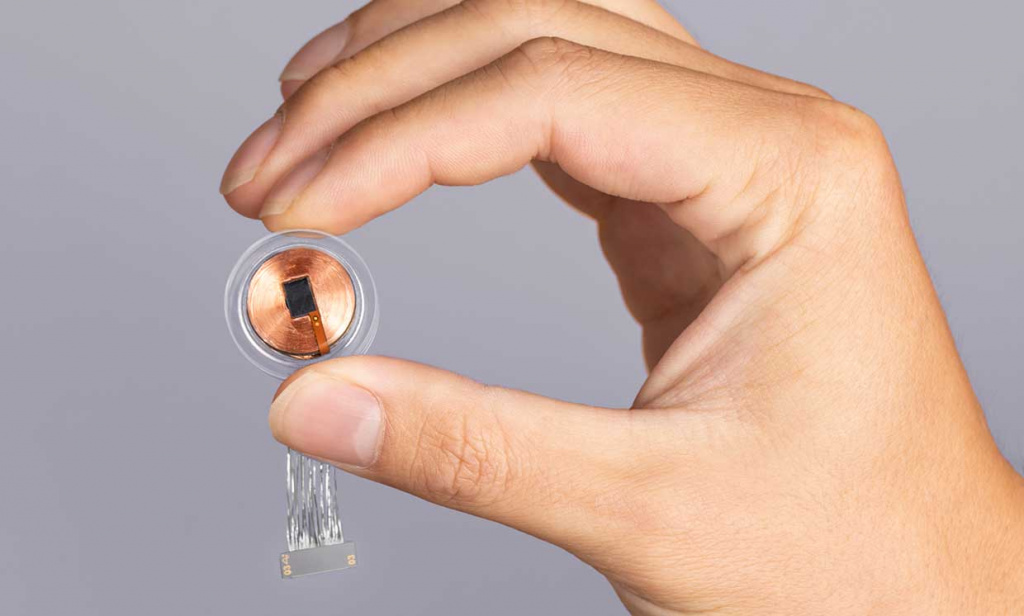
www.ginx.tv
Human with neuralink brain chip sees improvement after initial. Neuralink gets approval to put brain chip in humans. Elon musk's neuralink shows first brain-chip patient playing chess



:max_bytes(150000):strip_icc()/008_how-to-factory-reset-a-lenovo-laptop-5115817-a67348722ce94f9783881ea29e596310.jpg)